Dust generation in the pharmaceutical industry occurs during most bulk material handling steps, grinding, granulating, pelleting, coating, and even packaging. Due to the fine nature of this dust, it could quickly deflect and travel long distances to settle on surfaces and cracks in your facility.
Pharmaceutical Good Manufacturing Practices (GMP) are part of quality assurance that ensures that substances and drugs are produced and controlled to standards that match the requirements of their intended use.
In pharmaceutical production, no mistakes are possible, and an uncontrolled atmosphere can cause disasters. To reduce the risk of cross-contamination, mold and bacteria growth, and health risks to staff, each facility’s strict hygiene standards must cover the constant circulation of fresh, contaminant-free air.

Poor air quality can cause two types of problems:
Airborne contaminants can enter a facility through ventilation from the outdoors, air conditioning units, vents, or in airflow from other parts of the building. They can also come from materials used in the facility, such as compressed gas, cleaning and sterilizing agents, plastic, and stored materials.
An environment that is not carefully controlled can harm two critical aspects of pharmaceutical manufacturing:
1 – Product safety
Contaminants of all kinds can alter the effectiveness of substances and drugs, and make them ineffective or even dangerous.
This danger is greater in establishments that produce antibiotics in addition to other drugs. If cross-contaminated, traces of antibiotics can end up in other drugs and trigger allergic reactions in some patients who take them.
2 – Employee well-being
Personnel working in pharmaceutical production are exposed daily to dust, raw materials and potentially dangerous particles. During new product development, personnel may also work with nanoparticles that can be easily inhaled due to their microscopic size.
Drugs such as steroids, hormones and cytotoxics can be particularly harmful to people working on the production line and can gradually lead to lung inflammation or lung damage.
How to ensure clean air in your facility
Many pharmaceutical by-products contain harmful dust. This dust may contain Active Pharmaceutical Ingredients (APIs) such as narcotics, hormones, steroids, or other compounds that can have hazardous effects on employees or the environment if overexposed.
In an industrial technical report from the World Health Organization (WHO), ventilation temperature and relative humidity are recognized as essential design elements to avoid contamination and cross-contamination of products during manufacturing and storage.
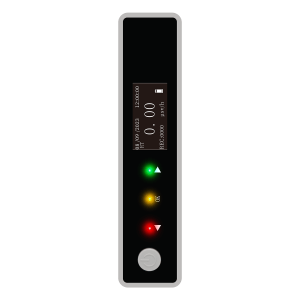
Filtration equipment with a powerful fan could play a role in the removal of dust, airborne particles, aerosols, fumes and eliminating bacteria almost anywhere in the premises, without disrupting sensitive production processes. Dust particle meters, on the other hand, could help you monitor 0.3um, 0.5um, 1.0um, 2.5um, 5.0um, and 10um particle size dust quantity concentration, temperature, and humidity. With data recording 999 groups, 3.2-inch TFT full-color display, automatic and manual measurement, real-time date and time, rechargeable lithium battery or separate external USB power supply, sensor life ≧ 8000 hours, data stability, and other features.
Current dust collectors are more autonomous and are often configurable to operate continuously. Equipment options allow dust collector controls to monitor pressures, regulate airflow, automatically pulse clean filters, and even discharge dust from the hopper without interrupting the process. However, even with high levels of automation, manual labor is required to keep dust collectors running efficiently. The most common of these tasks is replacing worn-out filters. Due to the toxic dust generated in pharmaceutical manufacturing, this filter replacement often requires a bag-in/bag-out (BIBO) process
Note: The bag-in/bag-out (BIBO) system improves filter changes and hopper dump maintenance to help reduce worker exposure to potentially hazardous dust, as well as exposure to contaminants present in the atmosphere.